Nitrates, nitrites, nitrosamines: latest research and history of preservatives
I recently learned that all nutritional supplements are periodically systematically re-evaluated. Previously accumulated research results are supplemented, and occasionally there is a revision of [tooltip tip=“Acceptable daily intake that can enter the human body every day throughout life, without harm to health.”]acceptable daily intake ADI[/tooltip]. In light of the latest revision of nitrite (E 249-250) and nitrate (E 251-252) from [tooltip tip=“European Food Safety Authority”]EFSA[/tooltip] has finally figured them out itself. In this review, I will try to objectively describe the benefits and harms of sodium nitrite and nitrates - the most demonized preservatives, their benefits and risks in terms of long-term consequences for human health.
If you are too lazy to read, at the end of the article there is a short summary of the article in the form of abstracts.
The material is based on evidence-based science and medicine. List of references, links and translations of sources at the end of the article.
Why are nitrates and nitrites added to foods?
Nitrite and nitrate salts are added to meat products as a preservative and antibacterial agent against botulinum toxin-producing microorganisms and other dangerous pathogens. As a bonus, the E-250 additive gives the product its characteristic taste and color.
Why meat? The ideal environment for Clostridium botulinum: absence of air, heat, humidity. For example, like in a sausage, or a jar of pickles. By the way, it is thanks to nitrites that industrial meat products have been trailing behind the list of sources of botulinum toxin poisoning over the past 50 years. Topping the list are homemade salted mushrooms.
The pink color that processed meat acquires is the result of the interaction of the myoglobin pigment with added nitrites - the nitric oxide formed from nitrite reacts with the pigment and converts it into another form: nitrosohemochrome.
 The pink color of processed meat is the result of NO3 reacting with the meat pigment.
The pink color of processed meat is the result of NO3 reacting with the meat pigment.
Not only Doctoral. Real sources of nitrates and nitrites
Vegetables and drinking water are the main sources of nitrates in the diet, and added preservatives account for no more than 5% of the total amount obtained “naturally”. Nitrates appear in water due to the work of microbes that oxidize ammonia in the soil. Sources of ammonia, in turn, are decomposing plants, manure, deposited automobile exhaust and combustion products, and nitrogen fertilizers.
Despite the widespread reduction in the use of nitrogen fertilizers, the amount of nitrates in groundwater is not reduced. Apparently, saltpeter is not the main source of pollution. By the way, tap water usually contains much less nitrates than from private wells.
A balanced diet rich in leafy vegetables may have significantly higher nitrate levels than normal, and this is normal.
I note that the level of nitrite in vegetables increases during storage, as nitrates are converted into nitrite (NO3 loses an oxygen molecule -> NO2), and in meat products, on the contrary, it decreases - being converted into nitric oxide (NO). More about this in the chemistry section.
The attitude towards these important additives is ambiguous - fears of preservatives are constantly fueled by the media, and not everyone wants to understand the issue more deeply.
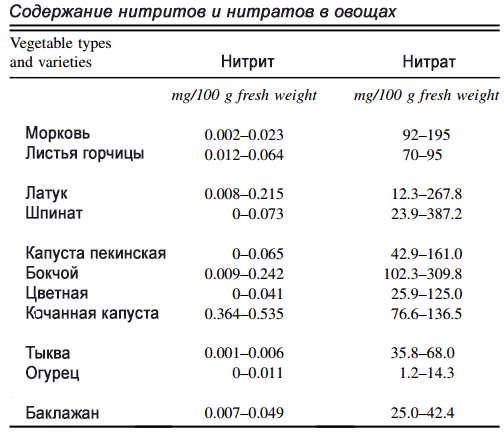
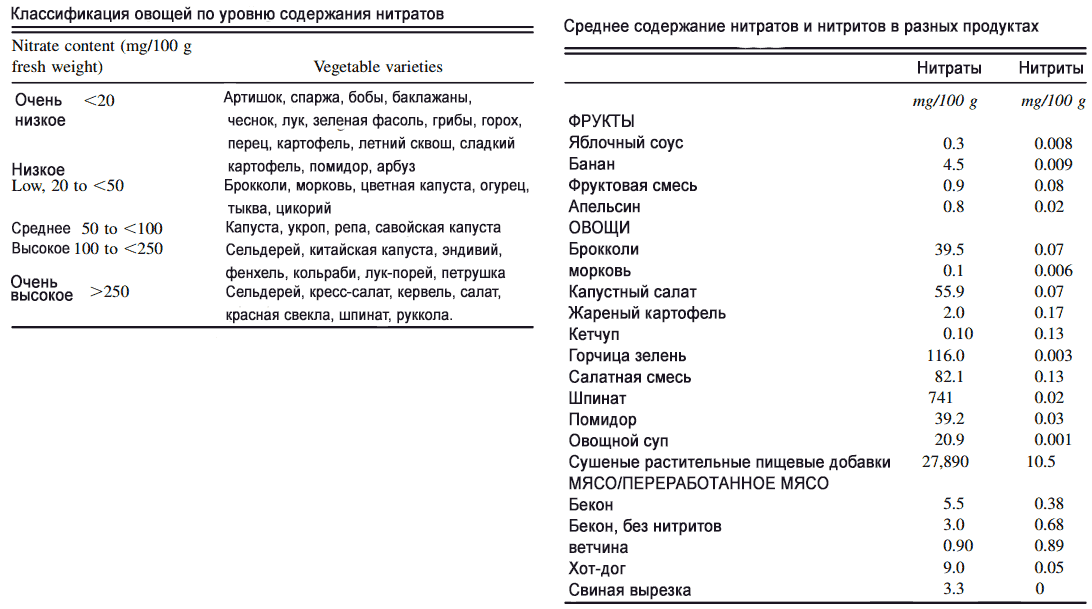
Nitrates in vegetables and fruits
Leafy green vegetables can contain more than 1000 mg of nitrates per kg of fresh greens. The record holders among vegetables are the plants we are used to: celery, lettuce (3500 mg/kg), beets, spinach (up to 4259 mg/kg), arugula, chard. Concentrations depend on the growing region, time of year, fertilizer application and plant variety. By comparison, processed meat products contain between 0.2 and 450 mg of nitrate per kilogram.
Read more about the nitrate content in food in the review Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions, in section 2.5 (links to texts in Russian at the end of the article); IARC monographs vol.94, pp. 46-100; Food sources of nitrates and nitrites: the physiological context for potential health benefits, Am J Clin Nutr 2009 90:1–10 American Society for Nutrition.
Let me give you an example. Beetroot juice lowers blood pressure and strengthens blood vessels. Let’s look at the numbers: two glasses of beet juice a day reduces systolic blood pressure from 5.4 to 12 mm Hg; diastolic - up to 10 mm Hg. This amount of beet juice contains from 154% to 630% of the daily dose of nitrates. Nitrate levels in a glass of organic beet juice range from 70% to 672% of the daily value; in inorganic - from 142% to 1260%.
These are just numbers; the amount of nitrates in itself does not tell us anything. Here’s why: The high levels of ascorbic acid, primary amines, and phenolic compounds in some fruits and vegetables inhibit the formation of other compounds from nitric oxide (NO), a precursor to nitrates, including nitrosamines. This property was studied and used to create a safe version of the E-250 additive (more details below).
The traditional Japanese diet contains an average of 18.8 mg/kg body weight of nitrates per day, with an ADI of 3.7 mg/kg. Studies on Europeans who were offered the Japanese diet under clinical supervision showed a decrease in diastolic pressure by an average of 5 units.
“Nitrate” plants are part of a balanced diet, and the food industry has not brought anything new, but has been able to control the negative properties of natural preservatives.
Regarding all kinds of devices, the so-called “nitrate meters”, read from Rospotrebnadzor .
Marketing paradoxes: celery instead of preservatives
In Canada and the USA, sausages that contain celery powder, a natural nitrate accumulator, are added instead of the E-250 additive. Sausages are advertised as healthier than those with chemically synthesized preservatives.
 The amount of nitrite in these sausages may even be higher than the regulated amount.
The amount of nitrite in these sausages may even be higher than the regulated amount.
Consumers who choose “green” foods add spinach, celery, and beet juice to their shopping carts, without realizing that these vegetables contain the same chemicals they avoid in processed meats (in quantities many times higher than normal for “chemical” preservative additives).
 Celery salt or celery powder is a supernitrite replacement for E-250, which increases the cost of the product several times.
Celery salt or celery powder is a supernitrite replacement for E-250, which increases the cost of the product several times.
The US Department of Agriculture strictly regulates the composition of products labeled “organic” and “natural” - the formulation should not include synthetic components. But in order for the product to remain tasty, beautiful and safe, preservatives and dyes still need to be added… in the form of celery powder or acerone cherry (so that the color is decent), mixed with a bacterial culture that converts nitrate into nitrite. The final organic product must contain no less concentration of the preservative E-250 than the non-organic product in order to be sold.
History of nitrate and nitrite from 200 BC. to this day
Meat was salted 5,000 years ago, but the first evidence of the use of nitrate salts was by the Romans around 200 BC (Homer has data from 850 BC). The Romans learned to salt meat from the Greeks, but it was they who first noted that evaporated salt from certain sources contributed to the intense pink color of the meat and enhanced its flavor.
 Roman bas-relief of the second century AD.
Roman bas-relief of the second century AD.
Much later, the salt “contaminant” was identified as potassium nitrate (formerly called nitrate). The chemical composition of nitrate was determined by Antoine Lavouzier himself. It is a pity that I cannot dwell on many historical events in more detail.
Before the Industrial Revolution, nitrates were obtained exclusively from natural sources: deposits around the world, from urine and ash, bat droppings, various organic matter and soil. Long before saltpeter was used in gunpowder, it was used to preserve meat and sausages. Procuring meat was an exact science that required experience and accuracy, since not only the taste and appearance of the product, but also the lives of consumers depended on the correct use of the preservative.
 Vintage treatises on meat preservation, 20s of the XX century.
Vintage treatises on meat preservation, 20s of the XX century.
In those days, when nitrate NO3 was the preservative, its conversion to nitrite NO2 was not always efficient, resulting in insufficient preservative effect, or inappropriately high levels of nitrates in the finished product.
Understanding of how saltpeter works came at the end of the 19th century. In 1891, Dr. Ed Polensky discovered the transition of nitrate to nitrite under the influence of certain types of bacteria. This observation changed the world, as it became clear that NO2 is responsible for the preservation and color of meat. At the same time, suppression of Clostridium botulinum, the main cause of severe botulinum toxin poisoning, was demonstrated.

The First World War made its own adjustments. The army needed well-stored canned goods, but ammunition was more important. The ban on the use of nitrate in the food industry in a number of countries in favor of the needs of weapons production forced butchers to switch to nitrite (more historical details [here]( https://earthwormexpress.com/the-history-and-use-of-nitrate-and- nitrite/bacon-and-the-art-of-living-15-concerning-the-direct-addition-of-nitrite-to-pork-curing-brine/)).
In 1923, a series of experiments began to determine the minimum level of sodium nitrite sufficient to effectively suppress bacteria and improve product quality. Sales of a huge military supply of sodium nitrite, or “Prague salt”, began. Traded to this day under the brand name “Powder Prague”.
There was also a “conspiracy”. Even before FDA approval, nitrite was secretly added as a preservative during 1905 in the United States.
WHO established the first ADI for nitrate in 1962. According to the FDA report on which the restriction was based, the WHO calculated that 0.5 grams of sodium nitrate per kg of body weight was safe for rats and dogs, and the rules were to divide this figure by 100 to ensure absolutely safe daily intake for humans - at 3.7 mg sodium nitrate per kg body weight.
The modern prejudice against these preservatives comes from the 60-70s, when animal studies showed the carcinogenic potential of nitrosamines (there will be a separate section on nitrosamines below).
A solution has been found. The recipe included antioxidants: vitamin E, sodium ascorbate or its isomer erythorbate, which prevent the formation of nitrosamines during heat treatment of meat. Despite this, the sharply negative attitude towards nitrite salt was perpetuated by the media, speculating on sensations and avoiding refutations.
In the 80s, the importance of nitric oxide and its metabolites in a host of physiological processes was realized, and the role of nitrate and nitrite was reconsidered. But the topic of “nitrite causes cancer” has been returned again and again for various reasons, with more and more evidence of the safety of supplements, but without convincing the general public. In the meantime, outbreaks of botulism have become extremely rare, and only thanks to nitrate-based preservatives.
If you are interested in the historical use of nitrite salt, read more: Nitrate and Nitrite – their history and functionality .
Chemistry of nitric oxide (NO), nitrites and nitrates
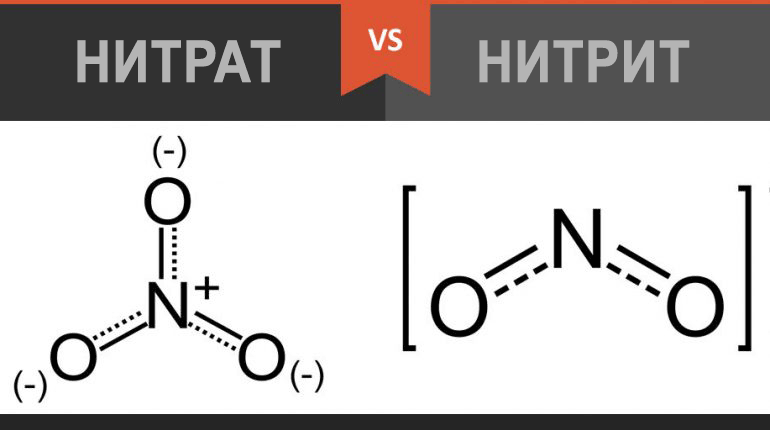
Nitrate NO3 is an ion ubiquitous in the environment. Formed from nitrogen monoxide (NO). NO is a natural compound that is synthesized in the body from the amino acid arginine, and also comes from outside with food and water.
Nitrate and nitrite are part of the nitrogen cycle, nitrate turns into nitrite when it loses one molecule of oxygen due to bacteria and other processes. The nitrogen cycle includes N-nitrosamines, N-nitrosamides and other nitrogenous compounds.
The role of nitric oxide in physiological processes is enormous. NO is a signaling molecule that can easily pass through the cell membrane and interact with receptor proteins, taking part in the “transmission of events” in the cell. The compound affects several processes simultaneously (pleiotropic signaling molecule).
What nitric oxide and its metabolites are responsible for:
- Regulates blood pressure and blood flow (remember IVs with nitrates in cardiology and nitroglycerin);
- Maintains the tone of blood vessels;
- Prevents platelets from sticking together;
- Participates in the transmission of nerve impulses and the energy process in mitochondria, in the functioning of the immune, endocrine systems and the retina;
- With its participation, vascular restoration after ischemia occurs faster, moreover, NO takes part in the relaxation of vascular smooth muscles;
- Reduces microvascular inflammation;
- Reduces oxidative stress;
- Stimulates the production of protective mucus in the gastrointestinal tract and increases blood flow in the gastric mucosa;
- Reduces the risk of type 2 diabetes and metabolic syndrome (so far proven only in laboratory animals).
- Currently, we are studying the effects of NO in the regeneration of the liver and heart muscle. Possible links to cystic fibrosis, hearing diseases and cluster headaches (the most common side effect of nitrate medications) are being studied.
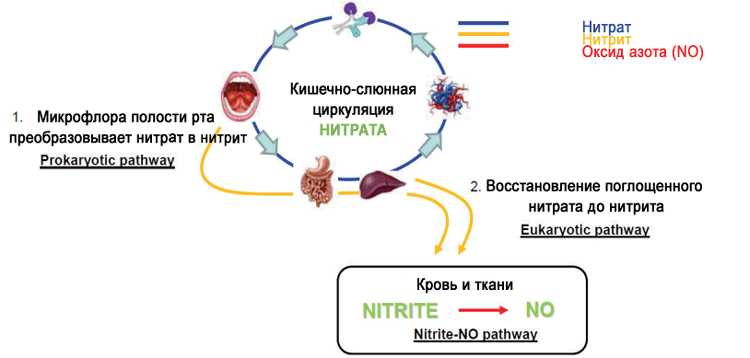
What happens to nitrates in the body
The biosynthesis of nitrates in the human body was first described in the 80s. It has been shown that nitrogen monoxide can be oxidized to nitrate and nitrite, and the latter can be partially reduced to active NO and found in the blood, urine and tissues.
Some of the nitrates absorbed with water and food are excreted unchanged. Bacteria in the oral cavity can capture some of the nitrate from food during chewing and convert it into nitrite (6-7%), which goes further through the salivary glands (up to 25%). NO3 levels in saliva can be 20 times higher than in plasma.
Why do we need a nitrate uptake mechanism? There is a theory, and some research to support it, that this is a form of immunity concentrated in the saliva and oral cavity: dietary NO3, converted to NO2, protects against external pathogens and those that can live in the aggressive environment of the stomach. In addition, from this stable nitrite (half-life 5-8 hours), the body can synthesize nitric oxide at any time when it is deficient (half-life from 0.05 to 1.18 ms).
Dietary nitrates provide an alternative source of nitrogen, in addition to arginine. Speaking of bacteria, rinsing the mouth after eating reduces the amount of nitrite in the plasma and slightly increases blood pressure in rats and humans.
Nitrite is part of the chemical composition of breast milk in the first days after birth. Moreover, infants receive almost 1 mg/kg bw per day, which is more than 10 times the ADI. Breast milk nitrite protects infants from pathogenic bacteria in the period before colonization by its microflora, which is capable of independently synthesizing NO2, and also acts as a source of nitric oxide, which prevents hypoxia.
The metabolism of compounds is affected by inflammatory processes in the body. Infections, parasites and autoimmune inflammatory diseases increase the biosynthesis of nitric oxide, nitrates and nitrites.
The level of NO2 in gastric juice is directly related to its acidity - if there is not enough acid, the growth of bacteria in the stomach increases, which reduce NO3. A complex chain reaction is started, leading to an increase in nitrate levels. Pathogenic bacteria in the kidneys and bladder can also do this.
Some of the reduced nitrogen compounds can increase the rate of mutations and [tooltip tip=“Programmed cell death, its self-destruction in case of infection with viruses, serious mutations and other reasons.”]apoptosis[/tooltip] cells, preventing hemoglobin from taking up oxygen. Negative effects directly depend on the amount of nitrates that enter the body from the outside or are synthesized by our microflora from nitric oxide.
Small amounts of nitrite can turn into a group of compounds called nitrosamines. Some nitrosamines have carcinogenic potential. The monograph [tooltip tip=“International Agency for Research on Cancer”]IARC[/tooltip] vol.94 describes in detail the biochemistry and pharmacology of nitrates and nitrites in section 4.1 Absorption, distribution, metabolism, and excretion.
The use of nitrates in medicine is described in the publication of the Swedish Institute of Pharmacology Inorganic And Organic Nitrates As Sources Of Nitric Oxide, section 1.3.1.
Nitrates, nitrites, nitrosamines and cancer
The role of nitric oxide and its derivatives in carcinogenesis has been actively studied for more than 50 years. Nitrites and nitrates do not themselves cause cancer, but they can form the carcinogenic compounds nitrosamines (detailed in the IARC report, section 4.3).
In 2010, the International Agency for Research on Cancer (IARC) listed nitrites in Group 2B. [/tooltip]: possibly carcinogenic to humans, together with “night shift work” and “diesel engine exhaust”. In the vast majority of studies, laboratory animals were exposed to nitrite through gavage or drinking water. Comparison with the control group does not show tumor growth ( report IARC). However, at the moment, the risk to humans is assessed in the totality of sources of nitrosamines, and not just through food - working conditions, smoking and other conditions are taken into account together.
The FDA has taken this potential exposure into account and has limited the permissible amount of nitrite to 700 ppm (0.07%) 1 . In addition, the addition of antioxidants erythrobate and ascorbate (they are “used” by plants in nature) prevents the formation of nitrosamines.
There are very few nitrites coming from outside to pose a health hazard. The main amount is synthesized in the body itself from other nitrogenous compounds. For most consumers, this is all they need to know about the safety of meat preservatives, but why not dig deeper!? Everything that science knows on this topic is collected in the IARC report vol.94 in sections 2-5.
The effect of nitrogen compounds on the body differs depending on the participation of certain catalysts, inhibitors, the presence of inflammatory processes, the pH of the environment, the number and type of bacteria capable of forming nitrite and nitrosamines from nitrate. This may be why studies on tumors often show completely opposite results (for such experiments, special breeds of rats have been bred with a predisposition to various forms of cancer that suffer for us).
Depending on the concentration of nitrogen and the type of tissue surrounding the tumor, nitrogen can either suppress the growth of mutated cells or provoke it. At high concentrations, N-nitroso compounds cause mutations and disruption of embryonic development in several animal species.
There is a correlation between an increased risk of colorectal cancer and high consumption of red meat and processed meats (I have not figured out what “high consumption” means). We only have access to epidemiological data; it is impossible to conduct full-fledged studies on humans. According to these data, the role of nitrogen compounds in carcinogenesis has not been conclusively confirmed.
A study of “tobacco” cancer in laboratory animals showed the carcinogenicity of nitrosamines, which are found in excess in tobacco and tobacco smoke. Nornicotine and nitrite are converted to N-nitrosonornicotine (NNN), a tobacco-specific nitrosamine carcinogen. It is not found in food or the environment, present only in tobacco smoke and some nicotine-based addiction treatment drugs. The relationship between the amount of N-nitrosonornicotine in a smoker’s urine and the risk of developing esophageal cancer is extremely high. If you smoke but don’t eat sausage because of E-250, then…
I present 2 long-term studies. Two-year observation of 100 rats divided into 3 groups, receiving 0%, 2.5 and 5% sodium nitrate of the total daily diet for 2 years from 8 weeks of life (equivalent to 0, 1259 and 2500 mg sodium nitrate per kg b.w. . in a day). Sufficient evidence for carcinogenicity has not been obtained.
Sodium nitrite was tested by the US National Toxicology Program for 2 years on mice and rats, 100 individuals in 4 groups. 0, 35, 70, or 130 mg sodium nitrite/kg bw was added to the water daily. males and 40, 80 or 150 mg sodium nitrite/kg b.w. to females. Proven carcinogenicity only in combination with amines and amides, some results in males were contradictory.
Nitrite and methemoglobinemia
Methemoglobinemia occurs when nitrite reacts with hemoglobin so that it can no longer carry oxygen. The disease occurs only in cases of severe poisoning through contaminated water or is completely congenital. The only case of an epidemic of methemoglobinemia was in the 50s, when cow manure with bacteria that converted nitrate into nitrite got into wells, and infant formula was prepared with this water. Anemia has never been directly associated with a preservative additive, and the disease is very rare.
The only reason meat products are safe
A huge influx of information and clickbait headlines confuse consumers. Food phobias, neuroses associated with fear of food and chemophobia are increasingly common phenomena. Meanwhile, outbreaks of botulism have become very rare thanks to E-250.
We have become too careless and superficial, refusing vaccinations and preservatives that save our lives every day, and at the same time overly cautious, limiting our diet and depriving ourselves of many useful substances. But to think about it seriously, you need a desire to know more than what is reported in the headlines.
It’s not at all difficult to completely give up sausages, Doctor’s and ham, but it’s worth remembering that we eat 95% of nitrites and nitrates with vegetables and water, and this is normal. The “natural” nitrite molecule and the one synthesized by man are identical and have no differences - we learned this in our first chemistry lessons at school. Don’t let anyone develop unreasonable fears in you!
Literature
The article is based on materials and publications of the European Food Safety Authority EFSA; Food Research Institute, University of Wisconsin USA; Molecular Nutrition & Food Research Journal; The American Journal of Clinical Nutrition; Oklahoma State University, Division of Agricultural Sciences and Natural Resources.
As per tradition, I made a machine translation for all materials and uploaded them to GoogleDrive . I recommend that you read the originals, since I couldn’t fit a lot of details into the article.
Google Drive contains the following documents:
- Dietary Nitrate and Nitrite: Benefits, Risks, and Evolving Perceptions (review from Food Research Institute, University of Wisconsin USA, 2016);
- EFSA explains risk assessment of nitrites and nitrates added to food (review from the European Food Safety Authority, timed to coincide with the planned review of additives, 2017);
- Nitrate and nitrite in the diet: How to assess their benefit and risk for human health (Molecular Nutrition & Food Research Journal, 2014) Food sources of nitrates and nitrites: the physiologic context for potential health benefits (The American Journal of Clinical Nutrition , 2009)
- Meat Curing (recommendations for processing meat with nitrite salt from an employee of the University of Oklahoma Frederick K. Ray Extension Animal Foods Specialist, with historical information and specific recipes).
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, VOLUME 94 Ingested Nitrate and Nitrite and Cyanobacterial Peptide Toxins, 2010.
- Food sources of nitrates and nitrites the physiological context for potential health benefits.
Briefly about nitrites
- Preservative sodium nitrite E-250 is the only approved additive that effectively inhibits the growth of bacteria that produce botulinum toxin.
- Under certain conditions, nitrite can form nitrosamines, which increase the risk of cancer. However, by adding sodium erythrobate (aka ascorbic acid or E-300) to the preservative, the process of converting nitrite into nitrosamine becomes impossible. In a word, nitrosamines are not synthesized from “sausage” nitrite.
- There is almost no nitrite left in the finished semi-finished meat product, since the compound is part of the nitrogen cycle. The additive cannot always be detected by laboratory tests.
- The dose of nitrite contained in a kilogram of fresh spinach can preserve 50 kg of ham.
- GOST 23670-79 for Doctor’s sausage, which was in force from 1981 to 2005, exceeded the permissible nitrite limit by 40%. This is a clarification for those who miss Soviet without chemistry.
- In the 21st century, nitrates are not added to meat, since the process of preservation with it takes several weeks, and with nitrite - 12 hours.
- Nitrite is the only reason why smoked meats, bacon, sausages, prosciutto, salami and other deli meats have not disappeared from the shelves.